IVD Wiki from Genrui
WHX Labs Dubai 2026, one of the most influential and large-scale exhibitions for medical laboratory equipment and supplies in the Middle East.Genrui proudly showcased its presence at this prestigious ...
Read More >From November 17th to 20th, MEDICA—the world’s largest and most influential event in the medical industry—was successfully held in Düsseldorf, Germany. Renowned for its professionalism, scale, and...
Read More >The 77th ADLM (Association for Diagnostics & Laboratory Medicine) was held in Chicago, USA, from July 29–31, 2025. As a global scientific and medical professional organization dedicated to clinic...
Read More >According to the latest news, Singapore has already made the registration of its local medical regulation authority, and it can be checked on the IMDRF list. See the official website below:
https://www.who.int/diagnostics_laboratory/EUL/en/

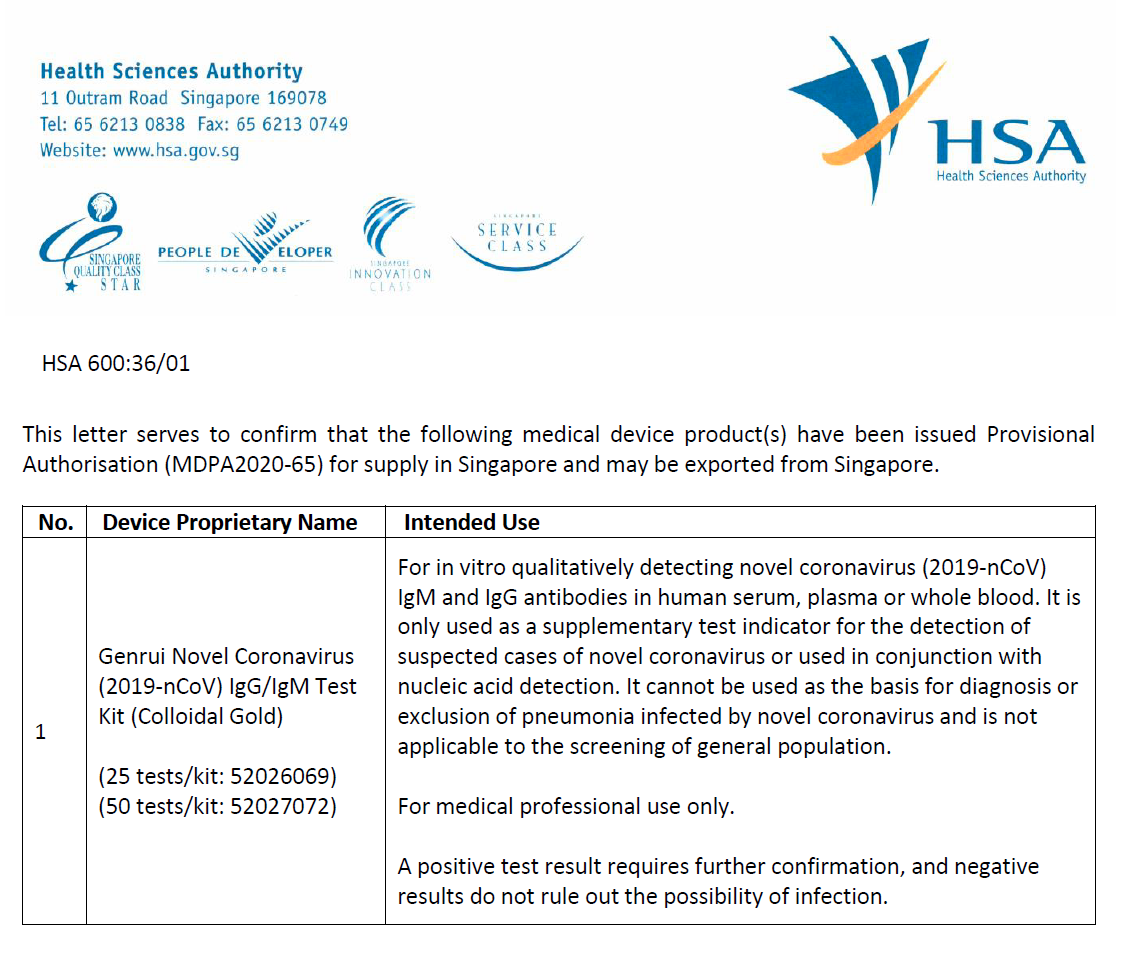
The International Medical Device Regulators Forum (IMDRF) was conceived in February 2011 as a forum to discuss future directions in medical device regulatory harmonization. It is a voluntary group of medical device regulators from around the world including the United States, Canada, Russia, the European Union, Japan, Australia, China, Korea, Singapore, Brasilia. WHO has been part of it as an observer since the Forum was founded.
The list has its positive effect as an approval of the product in the Singapore local market, more than that, it provides registration reference in other countries as well as WHO EUL list, which plays a significant part in many areas under the threat of a pandemic.
Now Genrui's product "Novel Coronavirus (2019-nCoV) IgG/IgM Test Kit (Colloidal gold)" is still on its way to serving more regions and areas to help people take control of disease as soon as possible.
